Tampa, Fla. (August 18, 2020) -- Medical device innovator PainTEQ hired Shanth Thiyagalingam as its
Chief Commercial Officer (CCO). PainTEQ is the creator of the LinQ™ SI Joint Stabilization System
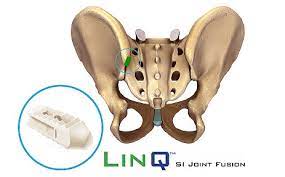
(pronounced “link”), a minimally invasive therapy for SI joint dysfunction.
Chief Commercial Officer (CCO). PainTEQ is the creator of the LinQ™ SI Joint Stabilization System
(pronounced “link”), a minimally invasive therapy for SI joint dysfunction.
Thiyagalingam oversees the company’s commercial strategy focused on sales, marketing and
professional education with the goal of increasing patient access to the LinQ procedure, which relieves
sacroiliac (SI) joint pain. Among various commercial initiatives, he will lead a training program to provide
instruction and certification for doctors who want to incorporate LinQ into their practice.
“Patient and physician satisfaction with the novel LinQ system indicates that PainTEQ is poised to
become the industry leader in treating chronic SI joint pain,” said Thiyagalingam. “We’re also fortunate
to be working with an impressive roster of clinicians and other experts advocating across the country
about the best-in-class benefits of LinQ.”
Thiyagalingam has nearly two decades of experience in the medical device and pharmaceutical
industries. He most recently worked at Abbott as General Manager APAC Neuromodulation, where he
led commercial efforts across Australia, Japan and Asia, targeted to alleviate chronic pain and
movement disorders. He has also held key management positions at Nevro, Stryker, and St. Jude
Medical.
“Shanth elevates our business to another level,” said PainTEQ CEO Sean LaNeve. “His clinical and
business acumen give us the capabilities of a top tier medical device company. His presence and
reputation in the market will allow us to scale our service to physicians and their patients across the
country and beyond.”
About PainTEQ: Built to bring interventional procedures to market, PainTEQ is a medical device innovator headquartered in Tampa, Florida. Working with pain management specialists to safely reduce and eliminate sacroiliac (SI) joint dysfunction, PainTEQ’s LinQ therapy is immediately providing clinical benefits to individuals living with incapacitating lower back pain, through a minimally invasive, FDA-approved, outpatient procedure.
About LinQ: The LinQ SI Joint Stabilization System provides SI joint dysfunction patients with a safe, minimally invasive solution to combat pain. After a thorough diagnostic process, physicians can help alleviate, and in many cases eliminate, chronic pain by placing a single LinQ allograft into the SI Joint. This single implant helps patients immediately regain joint stability – and with its large graft window, the LinQ SI Joint Stabilization System helps create an ideal environment for long-term fusion. Learn more at PainTEQ.com.
professional education with the goal of increasing patient access to the LinQ procedure, which relieves
sacroiliac (SI) joint pain. Among various commercial initiatives, he will lead a training program to provide
instruction and certification for doctors who want to incorporate LinQ into their practice.
“Patient and physician satisfaction with the novel LinQ system indicates that PainTEQ is poised to
become the industry leader in treating chronic SI joint pain,” said Thiyagalingam. “We’re also fortunate
to be working with an impressive roster of clinicians and other experts advocating across the country
about the best-in-class benefits of LinQ.”
Thiyagalingam has nearly two decades of experience in the medical device and pharmaceutical
industries. He most recently worked at Abbott as General Manager APAC Neuromodulation, where he
led commercial efforts across Australia, Japan and Asia, targeted to alleviate chronic pain and
movement disorders. He has also held key management positions at Nevro, Stryker, and St. Jude
Medical.
“Shanth elevates our business to another level,” said PainTEQ CEO Sean LaNeve. “His clinical and
business acumen give us the capabilities of a top tier medical device company. His presence and
reputation in the market will allow us to scale our service to physicians and their patients across the
country and beyond.”
About PainTEQ: Built to bring interventional procedures to market, PainTEQ is a medical device innovator headquartered in Tampa, Florida. Working with pain management specialists to safely reduce and eliminate sacroiliac (SI) joint dysfunction, PainTEQ’s LinQ therapy is immediately providing clinical benefits to individuals living with incapacitating lower back pain, through a minimally invasive, FDA-approved, outpatient procedure.
About LinQ: The LinQ SI Joint Stabilization System provides SI joint dysfunction patients with a safe, minimally invasive solution to combat pain. After a thorough diagnostic process, physicians can help alleviate, and in many cases eliminate, chronic pain by placing a single LinQ allograft into the SI Joint. This single implant helps patients immediately regain joint stability – and with its large graft window, the LinQ SI Joint Stabilization System helps create an ideal environment for long-term fusion. Learn more at PainTEQ.com.